Advanced Search
Lessons
- Unit Preview
- Plasma Vector Force Fields of Electric and Magnetic Fields
- Force on a Charged Particle
- Electric Field Calculations
- Static Electric Fields
- Magnitude of Force
- Electrical and Gravitational Potential Energy Problems
Force on a Charged Particle
We are well aware that a force acts between two or more charged objects. We've seen socks fresh from the dryer cling and we've discussed the forces on negative and positive charges. How do we define this force and how can we vary it?
The formula below describes the electric force between two charged objects and is known as Coulomb's Law.
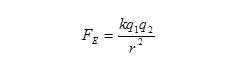
This law states that the force of attraction or repulsion between two charges is equal to the electrostatic constant, k, equal to 9.0 x 109, multiplied by the magnitude of the first charge, q1, multiplied by the magnitude of the second charge, q2, divided by the distance between the centers of the charges squared. It describes the force that one charge will exert on another.


